Cells rely on an intricate network of molecular chaperones and degradation systems to maintain proteostasis—the balance between protein synthesis, folding, and clearance. When this network fails, misfolded proteins can accumulate and ultimately drive neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease.
Our lab uses Caenorhabditis elegans and human cell models to uncover how the proteostasis network preserves cellular homeostasis and resilience throughout life and after stress or injury.
Here are our main projects:
1. The role of Molecular chaperones in alpha-synuclein and Tau propagation
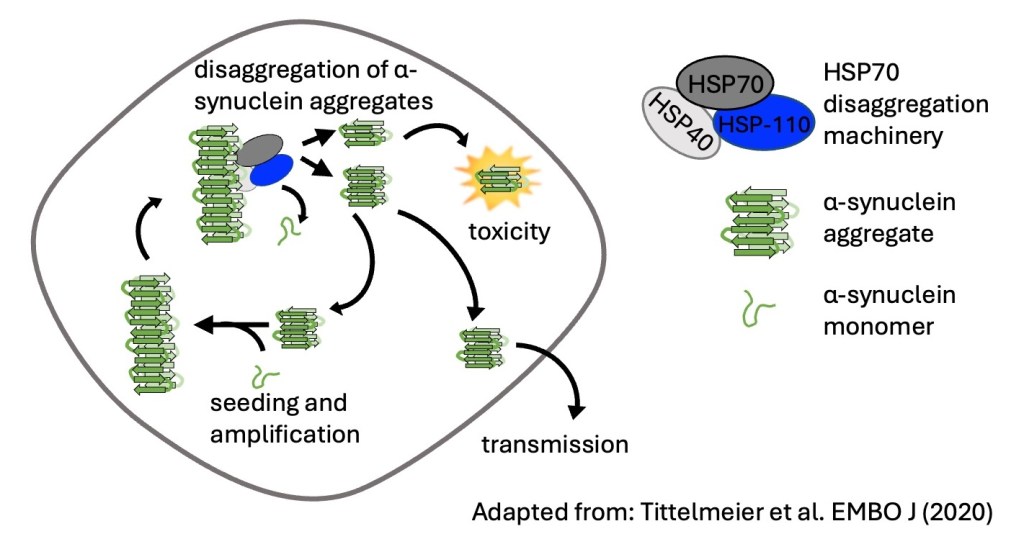
Molecular chaperones are central components of the proteostasis network, responsible for preventing protein misfolding and for refolding or disassembling misfolded proteins. However, the functionality of these quality control systems declines with age, which can lead to incomplete aggregate clearance and the accumulation of toxic degradation intermediates. We investigate how molecular chaperones influence the aggregation, disaggregation, and intercellular propagation of disease-associated proteins such as MAPT/Tau and α-synuclein (α-Syn).
Our work revealed that the HSP70 disaggregation machinery can have dual and context-dependent effects: while it can disaggregate and dissolve aggregates, it can also fragment amyloid fibrils, generating smaller seeding- and spreading-competent species that promote prion-like propagation (Nachman et al., JBC, 2020; Tittelmeier et al., EMBO J 2020; Tittelmeier, Nachman et al., Front Aging Neurosci, 2020). Using fluorescence lifetime imaging microscopy (FLIM), we further demonstrated that distinct α-Syn polymorphs are differentially processed by cellular clearance pathways, producing fibrillar species with enhanced seeding capacity (Tittelmeier et al., Commun Biol, 2022).
In our most recent study in collaboration with the Melki, Bukau, and Wentink labs (Jäger et al., EMBO J, 2025), we showed that α-Syn fibril polymorphism dictates how efficiently the HSP70 disaggregation machinery clears or fragments aggregates, demonstrating that the amyloid conformation itself determines whether chaperone activity is protective or detrimental.
2. Lysosomal Homeostasis
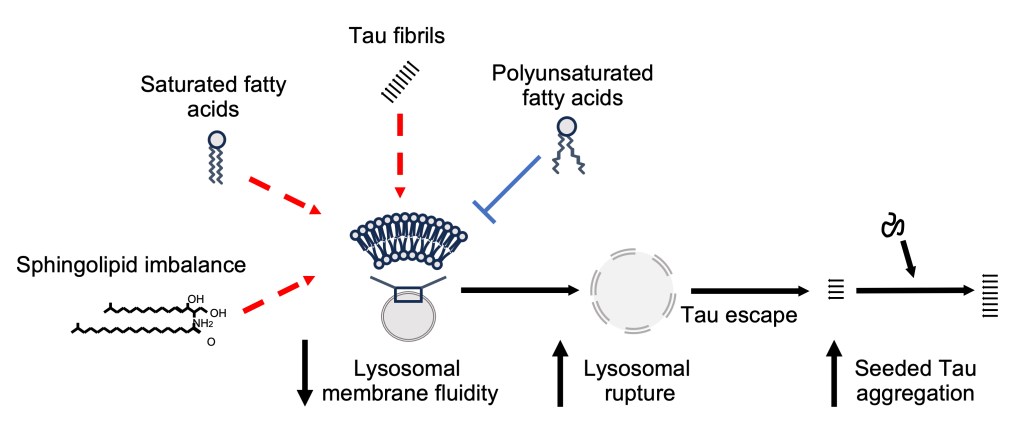
Once considered merely the cell’s “waste bag,” the lysosome is now recognized as a central hub that coordinates cellular metabolism, growth, and stress adaptation. As such, lysosomal health is essential for maintaining overall cellular function and organismal health. Our research investigates how cells preserve endolysosomal integrity and how lipid metabolism contributes to this process.
Using C. elegans models of α-Syn spreading, in which α-Syn is expressed in dopaminergic neurons or muscle cells, we demonstrated that both expression and chronic intercellular transmission of α-Syn cause endolysosomal membrane damage, mirroring observations in human neurons (Sandhof et al., Autophagy, 2020). To identify pathways that preserve endolysosomal integrity, we performed a genome-wide RNAi screen in a complementary C. elegans model for Tau spreading (Sandhof&Martin et al., Autophagy, 2025).
Functional characterization of the identified genes revealed that sphingolipid imbalance reduces endolysosomal membrane fluidity and compromises its stability, leading to vesicle rupture, enhanced seeded Tau aggregation, and neuronal toxicity (Tittelmeier et al., eLife, 2025). Remarkably, restoring membrane fluidity through unsaturated lipid supplementation prevented vesicle rupture and alleviated Tau-associated damage.
In our recent review article (Tittelmeier & Nussbaum-Krammer, Cells, 2025), we further discuss the interconnection between lipid and protein homeostasis, highlighting how disturbances in sphingolipid metabolism can affect proteostasis through lysosomal dysfunction.
3. Neuronal Vulnerability to Proteotoxic stress
We investigate how proteotoxic stress and early-life injury affect the long-term stability of the proteostasis network during aging and increase neuronal susceptibility to degeneration.
In our C. elegans models, the aggregation, spreading, and toxicity of disease-associated proteins intensify with age, reflecting a progressive decline in molecular chaperone capacity and endolysosomal resilience. We found that reduced insulin/IGF-1 signaling—through knockout of the DAF-2 insulin receptor, a well-established lifespan-extending intervention—helps maintain endolysosomal integrity and delay proteotoxicity (Sandhof et al., Autophagy, 2020).
Building on these findings, we are investigating how traumatic brain injuries (TBI) may contribute to the development of neurodegenerative diseases. Specifically, we study how mechanical injury affects proteostasis and how these acute cellular disturbances may ultimately lead to neurodegeneration. This work is part of an interdisciplinary collaboration between the German Armed Forces, BAM, and multiple groups at LMU Munich, combining expertise in weapons and explosives research, biomechanics, patient imaging, and molecular neurobiology.
4. Tissue- and cell type specific ribosome profiling

We have recently established a split-GFP–based system for tissue-specific ribosome profiling in C. elegans, enabling the selective tagging and isolation of ribosomes from individual tissues. This method not only opens new avenues to study intercellular transmission and cell type-specific toxicity of pathogenic proteins. It also enables the study of cell type-specific regulation of stress responses.

